Abstract
INTRODUCTION
In multiple myeloma (MM) the intensive chemotherapy strategy followed by autologous stem cell transplantation (ASCT) has improved the overall median survival of patients with MM from 36 months to more than 5 years in patients at standard risk. Despite modern therapy, most patients with MM will have disease progression. Several prognostic factors have been identified for the progression of the disease, such as beta-2-microglobulin, albumin (international staging system score), elevated serum lactate dehydrogenase and certain chromosomal abnormalities such as t (14; 16), 17 , 8q21 and the loss of 1p or gains of 1q. Among the main strategies to achieve the best response in the transplanted MM, is the improvement of the conditioning regimen.In some Latin American cities, access to melphalan IV is limited, the use of oral melphalan has been previously reported. The addition of bortezomib to high doses of melphalan has been reported by several groups and has shown that the toxicity profile Is comparable to the high doses of Melphalan (mel200).
OBJECTIVES
To assess the impact of bortezomib in combination with oral doses of oral melphalan (BorMel) on progression-free survival and overall survival of patients with multiple myeloma. We will also assess the toxicity and complications that occur during and after transplantation as well as time of grafting of both neutrophils and platelets.
METHODS AND PATIENTS
It is a unilateral, observational, descriptive study that included patients 18 to 70 years of age with diagnosis of multiple myeloma included in the program of autologous stem cell transplantation at the National Medical Center "20 de Noviembre" in the Hematology Service since 2010 to 2017. All of them received oral melphalan 200mg / m2SC conditioning from day -5 to -2, in addition to Bortezomib 1.3mg / m2SC on days -11, -8, -4, -1.
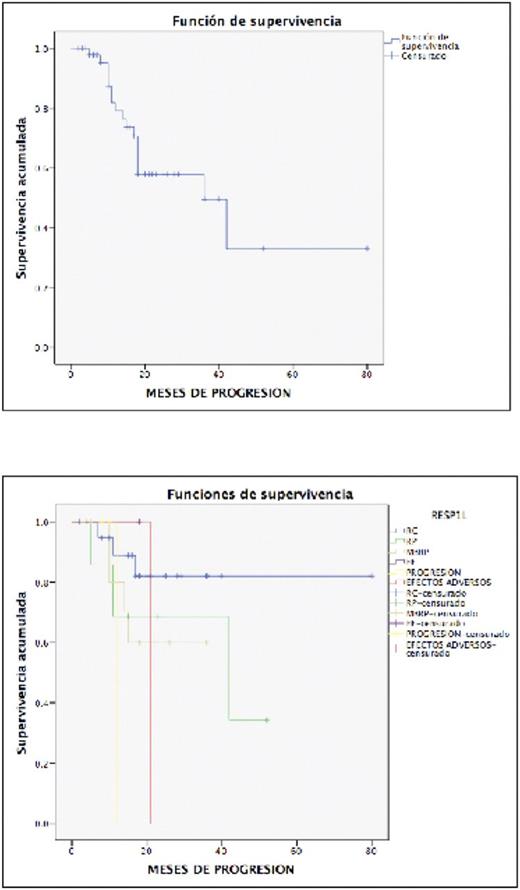
RESULTS
We included 47 patients, of whom 31 (65.9%) were men and 16 (34.1%) were women. The median age was 52 years (31-67 years).Regarding the immunoglobulin involved, 55% were IgG (26 patients), 12% IgA, non-secretors 8%. The Durie-Salmon stage I, II and III were 15, 14 and 71% respectively, 100% received first line bortezomib, the combination with Cyclophosphamide in 25%, Thalidomide 36% and Doxorubicin 29% mainly. To be admitted to ASCT, they had to have at least MBRP, 73% of the patients were transplanted after the first line of treatment, 17% required a second line, of these 4 (8.5%) by primary progression. The mobilization was based on Cyclophosphamide in 76.6%, the median of mononuclear cells obtained was 3.19 x 10(8) CMN / kg. There was no difference in the myeloid and platelet graft with mean of 13 days with a range of 9 to 24 days . During transplantation, 10% of the patients presented gastrointestinal toxicity, an infectious process in 40%, and the upper airway infection in 8%, the mortality during transplantation was 0%. 94% of patients entered maintenance treatment. At present, 63% of the patients continue to be maintained, progression-associated death in 6%, 17% in follow-up in other units, 25% progressed with an average of 18 months posttransplant.
CONCLUSIONS
The addition of bortezomib to oral melphalan proved to be safe, as it did not increase toxicity or transplant-related mortality, however, if there was an improvement in event- free survival when compared with previous studies of oral melphalan, where the mean was 14 months.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal